ADIPOQ
TEMPOL Offers Improved Treatment for Autoimmune Diseases
Matrix Biomed, Inc. provides a healthier, safer, and more sustainable option for control over TNF-α as a treatment for autoimmune diseases, through up regulation of ADIPOQ with TEMPOL. Typical therapies comprise anti-TNF agents such as adalimumab (trade name Humira) that target tumor necrosis factor alpha providing exogenous inhibition with serious and life-threatening side effects. Matrix Biomed, Inc.’s approach targets tumor necrosis factor alpha through endogenous regulation via the protein adiponectin without the same serious and life-threatening side effects. Here, TEMPOL upregulates the gene ADIPOQ that in turn codes for the protein adiponectin to positively regulate inflammatory responses. Accordingly, TEMPOL provides a better safer alternative treatment for autoimmune diseases such as Crohn’s, ulcerative colitis, rheumatoid arthritis, and inflammatory bowel disease among others.
ADIPOQ Overview
ADIPOQ is a gene that encodes for the protein Adiponectin. Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid oxidation. Adiponectin regulates numerous other proteins, genes, and biochemical pathways that are indicated in the pathogenesis of a variety of diseases. One such pathway that adiponectin regulates is the host inflammatory response. Of particular interest, is Adiponectin’s negative regulation of TNF-α. Discussed below is one pathway that adiponectin regulates, that in turn is modulated by the administration of TEMPOL.
ADIPOQ Regulates TNF-α
Tumor Necrosis Factor Alpha (TNF-α) is an inflammatory molecule and protein produced by the body’s cells. In healthy tissues and cells, TNF-α is an important immunologic surveillance cytokine necessary for proper immune system function, immune system response, and host defense. Cytokines are regulators of host responses to infection and inflammation. Some cytokines make diseases worse because they are pro-inflammatory while others reduce inflammation and promote healing because they are anti-inflammatory (Wojdasiewicz 2014). TNF-α plays a dual role providing both anti- and pro-inflammatory activity (Zakharova 2005).
The primary role of TNF is the regulation of immune cells. TNF-α may either promote the destruction of tissues or conversely promote tissue healing. TNF-α is able to induce fever, apoptotic cell death, cachexia, inflammation, inhibit tumorigenesis, inhibit viral replication, and respond to sepsis via IL1 & IL6 producing cells4. Standard treatments with anti-TNF agents do not distinguish between the different forms of TNF-α preventing both the body’s ability to eliminate harmful agents as well as the body’s ability to promote tissue healing.
Autoimmune disease are characterized by overexpression of TNF-α leading to excessive and prolonged activation of immune cells, such as T and B lymphocytes, overexpression of interlukin-6 (IL-6), interlukin-1 (IL-1), and interferon gamma (IFN-γ)4. In other words, overexpression of TNF-α causes the body’s own inflammatory response system to attack itself leading to a myriad of serious diseases and conditions. For example, these changes play a central role in the pathogenesis of autoimmune inflammatory responses in rheumatoid arthritis (RA), inflammatory bowel disease (IBD), Crohn’s disease (CD), neurological diseases, and many others.
Standard treatments for autoimmune diseases are known as anti-TNF agents that block or sequester TNF-α. As discussed above, TNF-α is necessary for proper host defense from infection and inflammation. Therefore, anti-TNF agents cause serious side effects by weakening the host immune system leaving the host defenseless against a myriad of diseases and conditions. Well documented examples of the serious side effects associated with anti-TNF agents include infusion reactions, infections, cardiac arrhythmias, demyelinating disorders, skin infections, and malignancies. Recognizing the danger these agents pose to the public, the U.S. Food and Drug Administration (FDA) issued a black box warning to doctors, which appears in the product labeling of TNF-inhibiting drugs, instructing them to increase measures to screen and monitor potential patients.
Despite these significant side effects, anti-TNF agents continue to dominate the pharmaceutical market ranking among the top 10 global block buster drugs. For example, the anti-TNF agents Humira (adalimumab), Remicade (infliximab), and Enbrel (etanercept) combined sales for the year 2015 were U.S. $30 Billion (Kuick Research 2016). Infliximab and adalimumab are in the subclass of “anti-TNF antibodies” and are capable of neutralizing all forms (extracellular-, transmembrane-, and receptor-bound) TNF-α . Etanercept is a fusion protein that reduces the effect of naturally present TNF, and hence is a TNF inhibitor, functioning as a decoy receptor that binds to TNF7. Despite various mechanisms of action, all of these anti-TNF agents work by suppressing TNF-α activity thereby predisposing patients to a significant list of serious side effects.
Through upregulation of ADIPOQ by TEMPOL, Matrix Biomed, Inc. provides a healthier, safer, and more sustainable option for control over TNF-α as a treatment for autoimmune diseases. Opposed to sequestering or blocking the body’s natural TNF-α, TEMPOL positively regulates TNF-α through upregulation of ADIPOQ. As shown below, ADIPOQ codes for the protein adiponectin that when increased, prevents or decreases the expression of TNF-α. As a testament to the drug development process at Matrix Biomed, Inc., their team focused research on discovering a compound or pathway that could provide natural regulation of TNF-α, as opposed to exogenous inhibition. Matrix Biomed, Inc.’s drug development process focuses on identification of genes of interest and regulation of that gene via upstream and/or downstream genes and their products. Through this type of natural regulation, TEMPOL reduces and or prevents over expression of TNF-α while also allowing TNF-α to respond, in necessary conditions, to infections and inflammation.
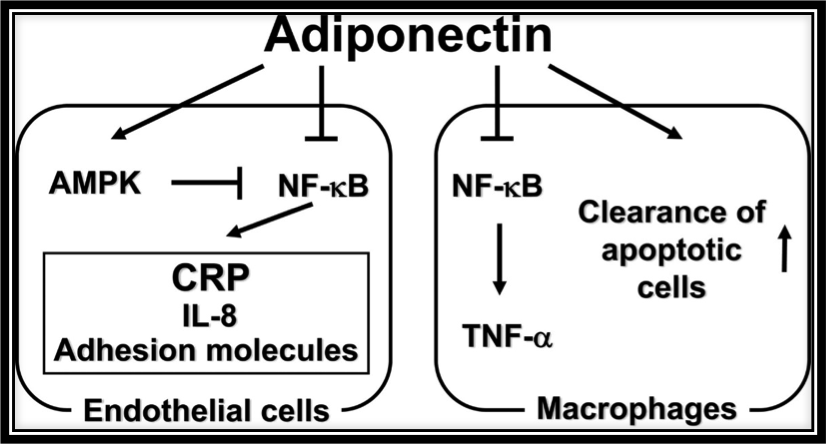
Matrix Biomed, Inc. discovered that TEMPOL increased the expression of ADIPOQ. ADIPOQ, in turn, codes for the adiponectin protein that naturally, in an endogenous manner, antagonizes or prevents TNF-α expression in two ways: 1) by negatively regulating its expression in various tissues such as liver and macrophages and 2) by counteracting the effect of TNF-α, as shown in the figure above. Furthermore, not discussed herein, adiponectin reduces inflammatory responses via multiples pathways, not just by reducing TNF-α expression. The figure above shows the anti-inflammatory actions of adiponectin. Adiponectin inhibits NF-κB activation in endothelial cells by activating AMPK which in turn inhibits NF-κB. By inhibiting NF-κB in endothelial cells, adiponectin effectively downregulates inflammatory response proteins C-reactive protein (CRP), IL-8, and adhesion molecule expression. In macrophages, adiponectin reduces inflammation by suppressing activation of NF-κB which in turn decreases TNF-α production while also promoting clearance of apoptotic cells by macrophages.
In numerous experimental models clinically and preclinically, researchers have shown a significant inverse correlation between plasma adiponectin and tumor necrosis factor alpha (TNF)-α mRNA expression. Subjects with the highest levels of adiponectin secreted the lowest levels of TNF-α. Furthermore, via inhibition of TNF-α, adiponectin effectively inhibits expression of IL-6 and IL-1 (Chen 2017).
By providing patients with natural endogenous regulation of TNF-α, patients will not be predisposed or subjected to the same serious adverse effects caused by anti-TNF agents. By using natural regulation of TNF-α, TEMPOL attenuates or prevents the host’s TNF-α from attacking itself while also allowing TNF-α to maintain its important function as an immunologic surveillance protein that responds to infection and inflammation.


